Mục Lục
What is the Law of Mass Action?
The law of mass action states that the rate of a reaction is proportional to the product of the concentrations of each reactant.
This law can be used to explain the behavior exhibited by solutions in dynamic equilibria. The law of mass action also suggests that the ratio of the reactant concentration and the product concentration is constant at a state of chemical equilibrium.
The Equilibrium Constant (Kc)
The concentration of reactants and products, at equilibrium, are constant at a given temperature. Consider the following simple reversible reaction where A & B are the reactants whereas C & D are the products.
A + B ⇌ C + D
A mixture of products and reactants in a state of chemical equilibrium is known as an equilibrium mixture. There exists a relation between the concentration of products and the concentration of reactants for an equilibrium mixture. This relation can be equated as follows.
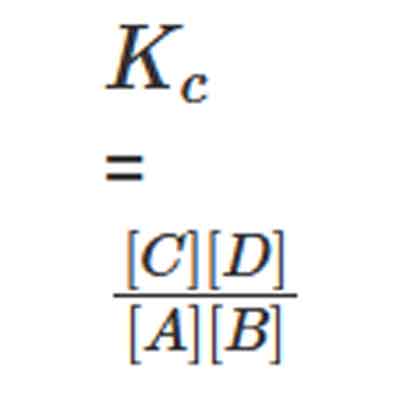
Here, Kc is called the equilibrium constant. In this equation, the concentration of A at equilibrium is represented as [A] (similarly for B, C, and D), and the stoichiometric coefficients of the reactants and products are 1. It has been experimentally observed that the equilibrium constant is also dependant on the stoichiometric coefficients of the reactants and products.
Therefore, the law of mass action dictates that the equilibrium constant, at a given constant temperature, is equal to the product of the concentration of products raised to the respective stoichiometric coefficients divided by the product of the reactant concentrations, each raised to the corresponding stoichiometric coefficient.
This is also known as the equilibrium law or the law of chemical equilibrium
Representation of the Equilibrium Constant
For a balanced reaction of the type,
aA + bB ⇌ cC + dD
According to the law of mass action, the constant value obtained by relating equilibrium concentrations of reactants and products is called the equilibrium constant. For the forward reaction, this is given by


Kc is the equilibrium constant expressed in terms of the concentration of the reactants/products. Similarly, Kp is the constant in terms of the partial pressures of the substances and K x is expressed in terms of the mole fraction.
Applications of the Law of Mass Action
This law is also applicable to semiconductors and, therefore, has several important implications in the fields of electronics and semiconductor physics. Here, the law of mass action provides a relationship between the concentrations of electron holes and free electrons when the semiconductor system is in a state of thermal equilibrium.
The law of mass action also has applications in the following fields:
- Mathematical ecology
- Sociophysics (social physics)
- Mathematical epidemiology
FAQs
1. What is mass law constant?
Ans: Mass action law states that a chemical reaction frequency is proportional to the active masses of reacting materials at a constant temperature. Kc is the reaction constant of equilibrium, while Kp is the constant of equilibrium found by applying partial pressure.
2. What are KP and KC?
Ans: Kc and Kp are the constants of the equilibrium of gas mixtures. The distinction between the two constants, however, is that Kc is determined by molar concentrations, while Kp is defined by the gasses ‘ partial pressures within a closed system.
3. What is the law of mass action examples?
Ans: Law of mass action, the law stating that the frequency of any chemical reaction is proportional to the sum of the masses of the reacting materials, each mass being elevated to a power equal to the coefficient in the chemical equation.
4. How the active mass is represented?
Ans: Usually active mass in units of mol dm3 is known to be the molar concentration; expressed as square brackets. [ ].
5. What is an effective concentration in chemistry?
Ans: Activity is a measure of a species ‘ active concentration under conditions that are not optimal (e.g., concentrated). This determines, rather than an idea, the real chemical potential for a real solution.
Learning Objectives
- Identify a system, an open system, a closed system and the environment of the system.
- Define a state of equilibrium.
- Describe the mass action law.
- Apply the mass action law to write expressions for equilibrium constants.
- Write the equilibrium constant expression for any reaction equation.
Heat is energy flowing from a high temperature object to a low temperature object. When the two objects are at the same temperature, there is no net flow of energy or heat. That is why a covered cup of coffee will not be colder than or warmer than the room temperature after it has been in there for a few hours. This phenomenon is known as equilibrium. In this example, we deal with the flow of energy.
Equilibria happen in phase transitions. For example, if the temperature in a system containing a mixture of ice and water is uniformly 273.15 K, the net amount of ice formed and melted will be zero. The amount of liquid water will also remain constant, if no vapor escapes from the system. In this case, three phases, ice (solid) water (liquid), and vapor (gas) are in equilibrium with one another. Similarly, equilibrium can also be established between the vapor phase and the liquid at a particular temperature. Equilibrium conditions also exist between solid phase and vapor phases. These are phase equilibria.
Chemical reactions may not be as complete as we have assumed in Stoichiometry calculations. For example, the following reactions are far short of completion.

Let us consider only the first reaction in this case. At room temperature, it is impossible to have pure NO2 or N2O4. However, in a sealed tube (closed system), the ratio
is a constant. This phenomenon is known as chemical equilibrium. Such a law of nature is called the law of mass action or mass action law.

Figure 1: A mixture of NO2 and N2O4 moves toward equilibrium. Colorless NO2 reacts to form brown NO2. As the reaction proceeds toward equilibrium, the color of the mixture darkens due to the increasing concentration of NO2.
Of course, when conditions, such as pressure and temperature, change, a period of time is required for the system to establish an equilibrium. Before we introduce the mass action law, it is important for us to identify a system or a closed system in our discussion. The law provides an expression for a constant for all reversible reactions. For systems that are not at equilibrium yet, the ratio calculated from the mass action law is called a reaction quotient Q. The Q values of a closed system have a tendency to reach a limiting value called equilibrium constant K over time. A system has a tendency to reach an equilibrium state.
A Closed System for the Equilibrium State
To discuss equilibrium, we must define a system, which may be a cup of water, a balloon, a laboratory, a planet or a universe. Thus, for discussion purposes, we define an isolated portion of the universe under consideration as a system, and anything outside of the system is called environment. When the system under consideration is isolated from its environment in such a way that there is no energy or mass transferred into or out of the system, the system is said to be an isolated system. In a isolated system, changes continue, but eventually there is no NET change over time; such a state is called an equilibrium state.
For example, a glass containing water is an open system. Evaporation lets water molecules escape into the air by absorbing energy from the environment until the glass is empty. When covered and insulated it is a closed system. Water vapor in the space above water eventually reaches an equilibrium vapor pressure. In fact, measuring of temperature itself requires the thermometer to be at the same state as the system it measures. We read the temperature of the thermometer when heat transfer between the thermometer and the system stops (at equilibrium). Equilibrium states are reached for physical as well as chemical reactions. Equilibrium is dynamic in the sense that changes continue, but the net change is zero.
Reversible Chemical Reactions
Heat transfer, vaporization, melting, and other phase changes are physical changes. These changes are reversible and you have already experienced them. Many chemical reactions are also reversible. For example
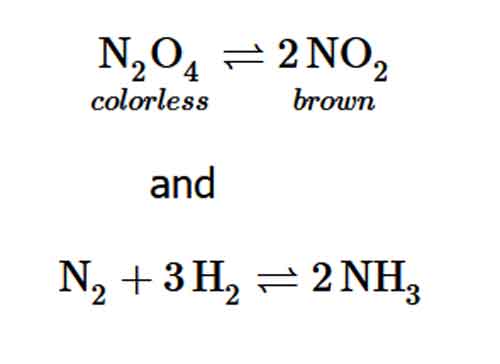
are reversible chemical reactions.
The Law of Mass Action
The law of mass action is universal, applicable under any circumstance. However, for reactions that are complete, the result may not be very useful. We introduce the mass action law by using a general chemical reaction equation in which reactants A and B react to give products C and D
.aA+bB→cC+dD
where a, b, c, d are the coefficients for a balanced chemical equation. The mass action law states that if the system is at equilibrium at a given temperature, then the following ratio is a constant:

The square brackets “[ ]” around the chemical species represent their concentrations. This is the ideal law of chemical equilibrium or law of mass action.
The Reaction Quotient Q vs. the Equilibrium Constants K
If the system is NOT at equilibrium, the ratio is different from the equilibrium constant. In such cases, the ratio is called a reaction quotient which is designated as Q.
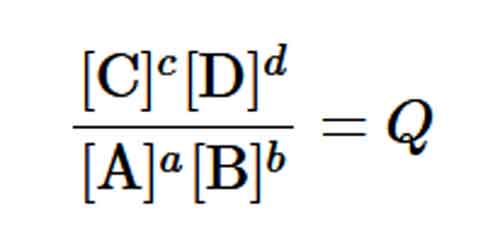
A system not at equilibrium tends to become at equilibrium, and the changes will cause changes in Q so that its value approaches the equilibrium constant,
K:Q→Keq
The mass action law gives us a general method to write the expression for the equilibrium constant of any reaction. At this stage, you should be able to write the equilibrium expression for any reaction equation. If you are not sure from the above general theory, here are some examples. It is more important for you to understand WHY the equilibrium constants are expressed this way than what the equilibrium expression is.
Example 1
Write the the equilibrium constant expression for the reaction equation:




The application of the mass action law leads to the method to write the expression for the equilibrium constant. The law is given in a general form, and these examples should help you grasp the method.
Questions
- A closed container has N2O4
and NO2 gases in it, and it has been placed in the lab for many days. What would you consider the container and the gases to be?
- an open system
- a closed system
- not a system
A closed container has N2O4 and NO2 gases in it, and it has been placed in the lab for many days. Is the system at an equilibrium state for the following chemical reaction?
N2O4⇌2NO2
A closed container has N2O4 and NO2 gases in it, and it has just been placed in an ice/water bath. Is the system at an equilibrium state for the following chemical reaction?
N2O4⇌2NO2
If the equilibrium constant for the reaction equation
HCOOH+CN−⇌HCN+HCOO−
is 5E5, what is the equilibrium constant for the reaction equation
HCN+HCOO−⇌HCOOH+CN−
?For reactions taking place in the gas phase, the equilibrium constant is normally expressed in terms of partial pressures of the reactants and products. If C represents the concentration, and other symbols of the ideal gas equation are used, which of the following is correct?

If Kc represents the equilibrium constant in terms of concentration, and Kp represents that in terms of partial pressure, which of the following is correct?

Solutions
- b
Discussion…
Since it has been in the lab, the temperature of the system is the same as its environment. - yes
Discussion…
Only a few minutes are required for the reaction to reach an equilibrium state. NO2
is a brown gas no
Discussion…
Heat will be extracted from the container, and that causes the equilibrium to shift. At 0 deg C, the equilibrium is shifted to have lots of N2O4. 1/5E5 = 2E-6
Discussion…
Since the reaction equation is reversed, use the relationship

Discussion…
Statements (c), (d), and (e) may be true for special cases, but they are not generally true. In this list, “Kc is proportional to Kp” is true, but a more quantative relationship will be derived. bd
Discussion…
Both reaction equations in (b) and (d) have equal numbers of reactants and products.
What is the Law of Mass Action?
The law of Mass action asserts that at a constant temperature, the product of the number of electrons in the conduction band and the number of holes in the valence band remains constant, regardless of the quantity of donor and acceptor impurities added.
It is expressed Mathematically as
np = ni2 = constant
Where ni is the intrinsic carrier concentration
n is number of electrons in the conduction band
p is number of holes in the valence band
The rate of each chemical reaction is proportional to the product of the Masses of the reacting substances, each Mass raised to a power equal to the coefficient that occurs in the chemical equation, according to the law of Mass action. The Norwegian scientists Cato M. Guldberg and Peter Waage created this law between 1864 and 1879, although it is now primarily of historical relevance. Although this law was beneficial for determining the correct Equilibrium equation for a reaction, the rate expressions it provided are now known to only apply to elementary reactions.
The Law of Mass Action in Practice
As this law also applies to semiconductors, it has a number of major consequences in the domains of electronics and semiconductor physics. When the semiconductor system is in thermal Equilibrium, the law of Mass action gives a link between the concentrations of electron holes and free electrons.
- The law of Mass action is also useful in the following areas:
- Ecological Mathematics
- Socio physics is a branch of physics that deals with (social physics)
Mathematical Expression of the Law of Mass Action
Consider a hypothetical reaction:
A + B → Products
As per the Law of Mass Action definition, the rate of reaction ‘R’ is given as:
R ∝ AB
For a general reaction denoted by aA + bB → Products, rate of the reaction according to the Law of Mass Action is indicated as:
R ∝ Aa Bb
As a result, Rf = Kf Aa Bb, where Kf is the rate constant of the forward reaction.
In the same way, the rate of backward reaction will be:
Rb ∝ Cc Dd
Now, in order for a reaction to being chemically balanced,
Rf = Rb
In the Law of Mass Action Equation, what is the Equilibrium Constant?
The Equilibrium constant, Kc, is defined as the ratio of the product of the Equilibrium concentrations of the products to the product of the Equilibrium concentration of the reactants in a balanced chemical equation, with each concentration term raised to the individual stoichiometric coefficients of the reactants and products.
The Law of Mass Action in a Gaseous System explained
The concentration terms are replaced with partial pressures in gaseous systems. We assume that the partial pressure of a gas is precisely proportional to its concentration at a given temperature to explain the Law of Mass Action for such systems.
The Equilibrium constant Kp in terms of partial pressures for the hypothetical reversible equation aA(g) + bB(g) cC(g) + dD(g) will be:
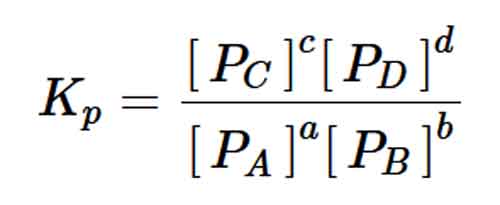
Ostwald’s Dilution Law is used to determine the dissociation Equilibrium of weak electrolytes using the Law of Mass Action.
- Semiconductor physics is a branch of physics that deals with semiconductors.
- For the purpose of explaining condensed matter diffusion
- In Mathematical epidemiology, to solve the disease transmission model.
Example
Consider the response. N2O4 2NO2
The Equilibrium constant for this reaction can be calculated using the Law of Mass Action formula:

The Law of Mass Action explains the relationship between the velocity of a chemical reaction and the molar concentration of the reactants at a particular temperature. Put forward by Norwegian scientists, Peter Wage and Cato Gulberg in 1864, the Law of Mass Action in Chemistry underpins many different types of physiological, biochemical, and pharmacological phenomena.
Now, let’s define the Law of Mass Action:
The law states that the rate of a chemical reaction at a given temperature and instant is directly proportional to the product of the reactants’ active Masses. Here, active Mass means the molar concentration of a substance per unit volume of it. The unit of active Mass is mol dm-3, and its value is expressed within square brackets.
Mathematical Expression of the Law of Mass Action
Consider a hypothetical reaction:
A + B → Products
As per the Law of Mass Action definition, the rate of reaction ‘R’ is given as:
R ∝ A B
For any general reaction denoted by aA + bB → Products, the rate of the reaction according to the Law of Mass Action is indicated as:
R ∝ Aa Bb
How to Derive the Law of Mass Action?
Consider the following reversible reaction hypothetically:
aA + bB ⇌ cC + dD
According to the Law of Mass Action, the rate of forward reaction will be:
Ri ∝ Aa Bb
Therefore, Ri = Kf Aa Bb , where Kf is the forward reaction’s rate constant
Similarly, the backward reaction rate will be:
Rb ∝ Cc Dd
Or, Rb = Kb Cc Dd , where Kb is the backward reaction’s rate constant
Now, for a reaction to be in chemical Equilibrium,
Rf = Rb
Or Kf Aa Bb = Kb Cc Dd

The above equation is known as the Mass Law equation, and Kc is termed as the Equilibrium constant.
What is the Equilibrium Constant in the Law of Mass Action Equation?
Kc or the Equilibrium constant is expressed as the ratio of the product of the Equilibrium concentrations of the products to the product of the Equilibrium concentration of the reactants, with each concentration term raised to the individual stoichiometric coefficients of the reactants and the products in the balanced chemical equation.
Explanation of the Law of Mass Action for a Gaseous System
For gaseous systems, the concentration terms are replaced by partial pressures. To explain the Law of Mass Action for such systems, we consider that the partial pressure of a gas is directly proportional to its concentration at a given temperature.
For the hypothetical reversible equation aA(g) + bB(g) ⇌ cC(g) + dD(g), the Equilibrium constant Kp in terms of partial pressures will be:

Concentration Quotient versus Equilibrium Constant
The Concentration Quotient (Qc)of a chemical reaction at a given temperature is defined as the ratio of the product of the concentrations of the products to that of the reactants. However, as the system reacts the value of Qc will fluctuate, but the Equilibrium concentrations will determine the Equilibrium constant Kc.
If Qc > Kc, the reaction will be occurring in the backward direction
If Qc < Kc, the reaction will be occurring in the forward direction
If Qc = Kc, the reaction shall remain in Equilibrium
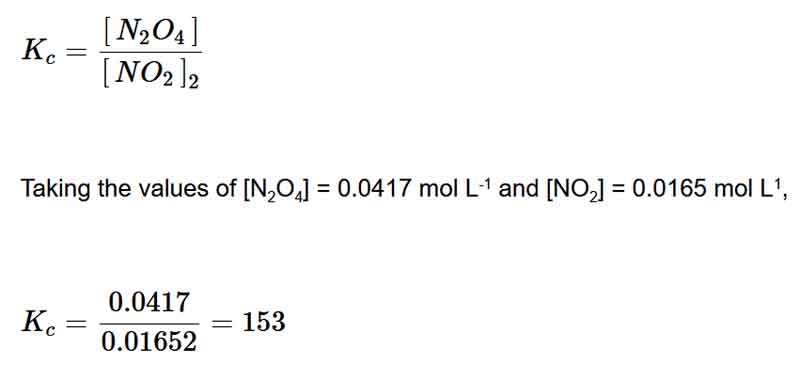
Example of the Law of Mass Action
Consider the reaction 2NO2 ⇌ N2O4
Applying the Law of Mass Action formula, the expression for the Equilibrium constant for this reaction is:
Applications of the Law of Mass Action
- Ostwald’s Dilution Law for determining the dissociation Equilibrium of weak electrolytes.
- Semiconductor physics.
- For explaining diffusion in condensed matter.
- For solving the model of disease dissemination in Mathematical Epidemiology.
FAQs (Frequently Asked Questions)
1. What are the Factors Affecting the Rate of Chemical Reactions?
- Concentration: The reaction rate increases with an increase in the concentration of reactants.
- Pressure: Pressure influences gaseous reactions. These can be categorised as three types of gaseous reactions:
- Reactions With an Increase in Volume: For reactions that are accompanied by an increase in volume, a reduction in pressure increases the rate of reaction.
Example: PCl5(g) ⇌ PCl3(g) + Cl2(g)
- Reactions With a decrease in Volume: For reactions that are accompanied by a reduction in volume, an increase in pressure increases the rate of reaction.
Example: N2(g) + 3H2 ⇌ 2NH3 (g)
- Reactions with No Change in Volume: Reactions, where volume remains unchanged are independent of changes in pressure.
Example: H2(g) + Cl2 ⇌ 2HCl (g)
- Temperature: In general, reaction rates increase with an increase in temperature.
- Catalysts: Catalysts increase the reaction rate.
- Particle size: Solid reactants in powdered form provide a greater surface area to increase the reaction rate.
2. What is Le Chatelier’s Principle of Chemical Equilibrium?
Le Chatelier’s principle states that for reactions that are in dynamic equilibria, changes in concentration, pressure, or temperature shifts the position of Equilibrium to counteract the change and to establish a new Equilibrium state.
- Concentration:Increasing the reactant concentration of a system will shift the Equilibrium towards the right, forming more products. Conversely, reducing the concentration of the product(s) will also shift the Equilibrium towards the right.
- Pressure: If the volume of a system decreases, or pressure increases, the Equilibrium will shift in the direction that involves a lesser number of moles of gas. Similarly, if the pressure decreases and the volume of the system increases, the Equilibrium will favour the side that produces more number of moles of gas.
- Temperature: For endothermic reactions, an increase in temperature will shift the Equilibrium to the right. Conversely, for exothermic reactions, increasing the temperature will shift the Equilibrium to the left.
Xem thêm

Để lại một phản hồi